Wang, Y., Fan, M., Wang, H., You, Y., Wei, C., Liu, M., Luo, A., Xu, X., & Duan, X. (2022). Relative safety and efficacy of topical and oral NSAIDs in the treatment of osteoarthritis: A systematic review and meta-analysis. Medicine, 101(36), e30354. https://doi.org/10.1097/MD.0000000000030354
Abstract
Background: Osteoarthritis (OA) often affects the hands, knees, and hip joints, causing considerable pain and disability, and often affecting the patient’s quality of life. Non-steroidal anti-inflammatory drugs (NSAIDs) are common pain relievers often applied as first line therapies for OA. However, prolonged NSAIDs application can have unwanted side effects. Given this, this study was designed to systematically evaluate the efficacy and safety of topical and oral NSAIDs for the treatment of OA.
Methods: We searched the PubMed, Embase, Cochrane Library, and Web of Science databases for relevant papers from their inception dates to May 2021. Our study only included randomized controlled trials comparing topical and oral NSAIDs and all data were analyzed using Review Manager version 5.3 (RevMan version 5.3).
Results: We identified 8 RCTs (2096 patients with OA), for evaluation and revealed that, in general, topical and oral NSAIDs presented with similar efficacies for the treatment of OA. The Western Ontario and McMaster Osteoarthritis Index for assessing pain relief in OA patients was (standardized mean difference [SMD] 0.07; 95%CI −0.02, 0.17) and visual analog scale was (SMD −0.01; 95%CI −0.02, 0.18), and improved stiffness in OA patients (SMD 0.09; 95%Cl 0.03, 0.20).
Conclusions: Topical NSAIDs are as effective as oral NSAIDs for the treatment of OA and both topical and oral NSAIDs are equally effective in reducing pain and improving physical function in OA patients. In terms of safety, a larger number of samples are still needed to determine if there are any differences in the safety profile of topical or oral NSAIDs.
Effectiveness and safety of non-steroidal antiinflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis
da Costa, B. R., Pereira, T. V., Saadat, P., Rudnicki, M., Iskander, S. M., Bodmer, N. S., Bobos, P., Gao, L., Kiyomoto, H. D., Montezuma, T., Almeida, M. O., Cheng, P. S., Hincapié, C. A., Hari, R., Sutton, A. J., Tugwell, P., Hawker, G. A., & Jüni, P. (2021). Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ (Clinical research ed.), 375, n2321. https://doi.org/10.1136/bmj.n2321
Abstract
Objective To assess the effectiveness and safety of different preparations and doses of nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, and paracetamol for knee and hip osteoarthritis pain and physical function to enable effective and safe use of these drugs at their lowest possible dose.
Design Systematic review and network meta-analysis of randomised trials.
Data sources Cochrane Central Register of Controlled Trials (CENTRAL), Medline, Embase, regulatory agency websites, and ClinicalTrials.gov from inception to 28 June 2021.
Eligibility criteria for selecting studies Randomised trials published in English with ≥100 patients per group that evaluated NSAIDs, opioids, or paracetamol (acetaminophen) to treat osteoarthritis.
Outcomes and measures The prespecified primary outcome was pain. Physical function and safety outcomes were also assessed.
Review methods Two reviewers independently extracted outcomes data and evaluated the risk of bias of included trials. Bayesian random effects models were used for network metaanalysis of all analyses. Effect estimates are comparisons between active treatments and oral placebo.
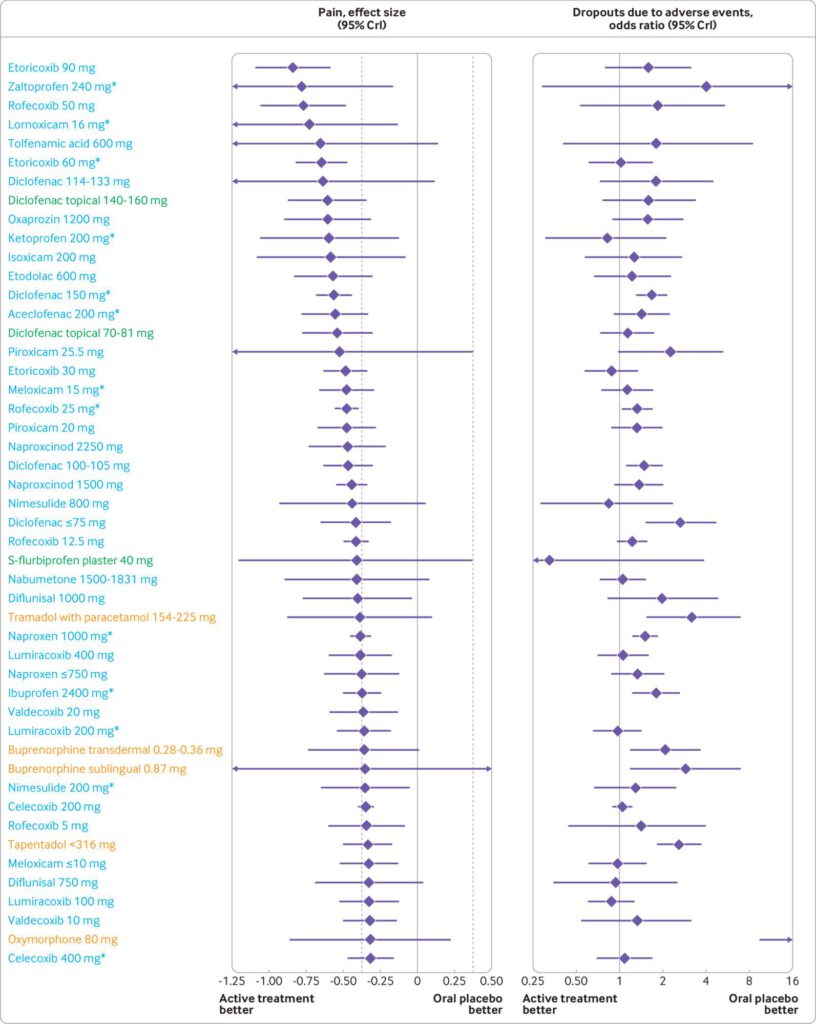
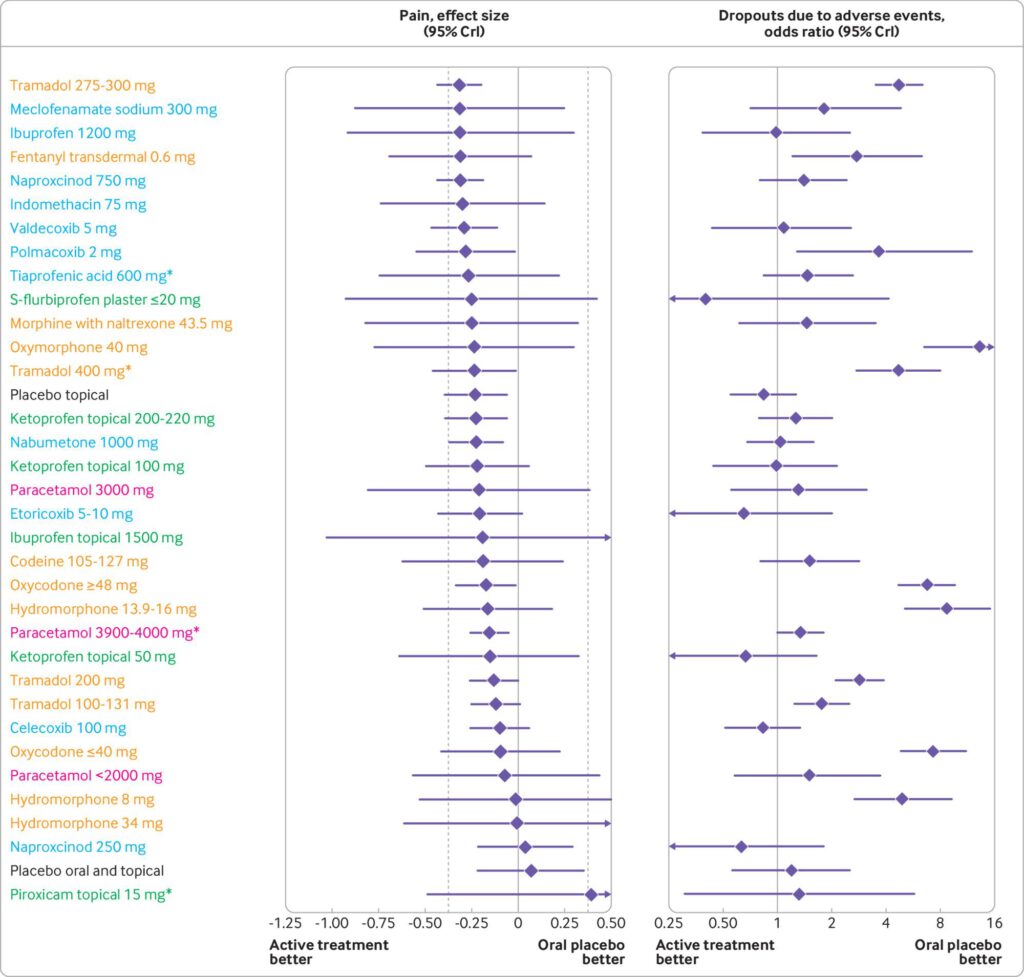
Results 192 trials comprising 102 829 participants examined 90 different active preparations or doses (68 for NSAIDs, 19 for opioids, and three for paracetamol). Five oral preparations (diclofenac 150 mg/day, etoricoxib 60 and 90 mg/day, and rofecoxib 25 and 50 mg/day) had ≥99% probability of more pronounced treatment effects than the minimal clinically relevant reduction in pain. Topical diclofenac (70-81 and 140-160 mg/day) had ≥92.3% probability, and all opioids had ≤53% probability of more pronounced treatment effects than the minimal clinically relevant reduction in pain. 18.5%, 0%, and 83.3% of the oral NSAIDs, topical NSAIDs, and opioids, respectively, had an increased risk of dropouts due to adverse events. 29.8%, 0%, and 89.5% of oral NSAIDs, topical NSAIDs, and opioids, respectively, had an increased risk of any adverse event. Oxymorphone 80 mg/day had the highest risk of dropouts due to adverse events (51%) and any adverse event (88%).
Conclusions Etoricoxib 60 mg/day and diclofenac 150 mg/day seem to be the most effective oral NSAIDs for pain and function in patients with osteoarthritis. However, these treatments are probably not appropriate for patients with comorbidities or for long term use because of the slight increase in the risk of adverse events. Additionally, an increased risk of dropping out due to adverse events was found for diclofenac 150 mg/day. Topical diclofenac 70-81
mg/day seems to be effective and generally safer because of reduced systemic exposure and lower dose, and should be considered as first line pharmacological treatment for knee osteoarthritis. The clinical benefit of opioid treatment, regardless of preparation or dose, does not outweigh the harm it might cause in patients with osteoarthritis.
Conflict of interest statement
support from the Arthritis Society, Canada Research Chairs Programme, National Institute for Health Research, Chevening Scholarship Program for the submitted work. PJ serves as unpaid member of the steering group of trials funded by Appili Therapeutics, Abbot Vascular, and Terumo; he has received research grants to the institution from Appili Therapeutics, and honorariums to the institution for participation in advisory boards or consulting from Amgen, Ava and Fresenius, but has not received personal payments by any pharmaceutical company or device manufacturer. AJS has been a paid consultant by Janssen-Cilag and GlaxoSmithKline. PT has received royalty payments as contributing author and editor for journals, textbooks and articles published by Elsevier, Little Brown, Wolters Kluwer, and John Wiley and Sons; PT has been an independent paid consultant to CHEOR Solutions (Canada), Innovative Science Solutions LLC and Reformulary Group; he serves as unpaid chair of the management subcommittee, of the executive committee of OMERACT; OMERACT receives unrestricted educational grants from the American College of Rheumatology, European League of Rheumatology, Amgen, Astra Zeneca, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech/Roche, Genzyme/Sanofi, Horizon Pharma, Merck, Novartis, Pfizer, PPD, Quintiles, Regeneron, Savient, Takeda Pharmaceutical, UCB Group, Vertex, Forest and Bioiberica; PT serves as an independent committee member in Data Safety Monitoring Boards of FDA approved trials being conducted by UCB Biopharma GmbH and SPRL, Parexel International and Prahealth Sciences. All other authors report no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. All authors report no other relationships or activities that could appear to have influenced the submitted work.
Fazit:
Bei oberflächennahen Gelenken scheint das topische NSAR tatsächlich der oralen Gabe hinsichtlich der Wirkung gleichwertig zu sein bei weniger systemischen Nebenwirkungen.